Characteristics of Canal Rays :
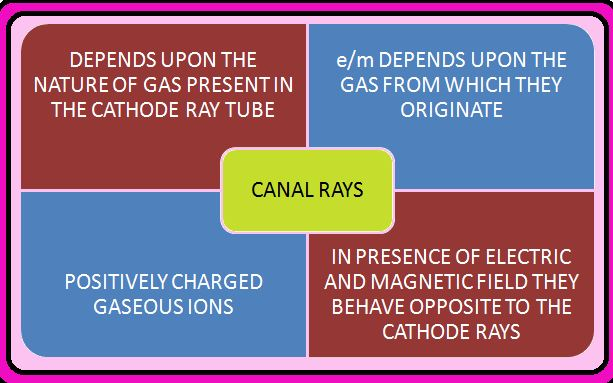
(i) The positively charged particles depend upon the nature of gas present in the cathode ray tube. These are positively charged gaseous ions.
(ii) The charge to mass ratio of the particles is dependent on the gas from which these originate.
(iii) Some of the positively charged particles carry the multiple of the fundamental electrical charge unit.
(iv) These particles in the magnetic or electrical field behave opposite to the cathode rays or electron.
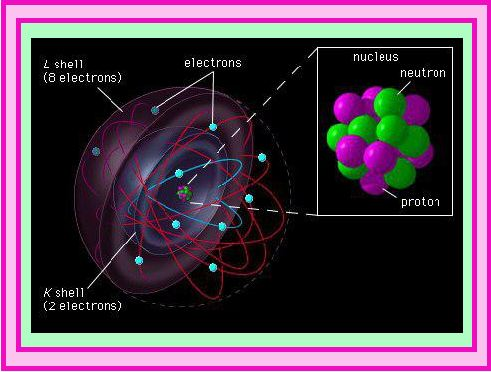
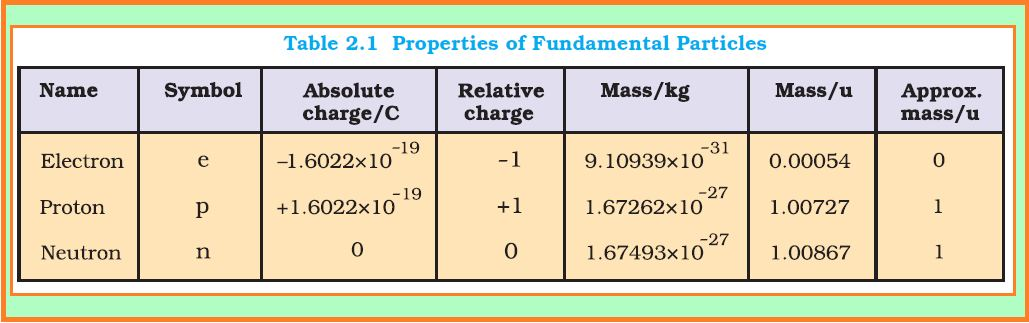
The smallest and lightest positive ion was obtained from hydrogen and was given the name proton by Rutherford.
(ii) The charge to mass ratio of the particles is dependent on the gas from which these originate.
(iii) Some of the positively charged particles carry the multiple of the fundamental electrical charge unit.
(iv) These particles in the magnetic or electrical field behave opposite to the cathode rays or electron.
The smallest and lightest positive ion was obtained from hydrogen and was given the name proton by Rutherford.